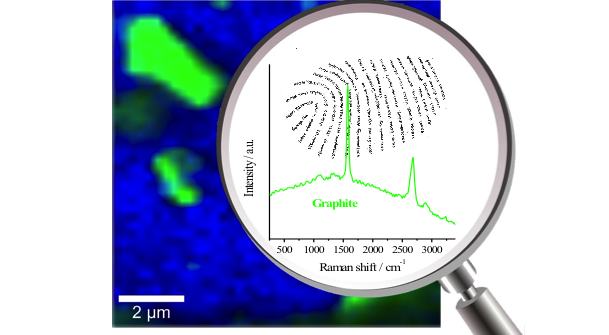
As discussed in previous posts, Raman spectroscopy is a rapidly growing analytical technique used in a wide variety of industries for material identification, but with so many different laser options it can be somewhat challenging to understand which laser is best for which application. To help elevate some of the confusion around this issue, we released an application note this past August titled “Multi-Mode vs. Single-Mode Lasers for Raman Spectroscopy,” in which we explained the pros and cons of these two categories of lasers, and made recommendations for which is preferable in different applications. In this blog post, we are going to take a look at another essential aspect for when selecting which laser to use, the wavelength. This post is by no means exhaustive and doesn’t explore things such as resonance effects or surface plasmonics, but provide you with the fundamentals you need to quickly determine which laser wavelength is best for most common Raman applications.
Raman spectroscopy measures the vibrational modes structure of a molecule by collecting inelastically scattered laser light and calculating the frequency difference between the scattered light and the excitation laser. This shift results from the photon inelastically scatters off of the molecule, where it excites the vibrational mode transferring energy to the molecule and therefore the photon losses energy which are detected as a shift in wavelength. Because Raman spectra are calculated based on the energy difference between the incident and scattered light, this means that the Raman effect is independent of the laser wavelength. While this is true, there are a wide variety of practical considerations that need to be understood when choosing your laser wavelength. The three main factors when selecting your excitation wavelength are scattering efficiency, auto-fluorescence, and detector availability. So while this blog post is not intended to go into the physics behind any of these three properties, it should serve general guidance when deciding which wavelength to choose.
Just as in the more familiar case of Rayleigh scattering, the efficiency of the Raman effect is inversely proportional to the wavelength raised to the 4th power. Meaning that for every 16% the laser wavelength is reduced the number of photons inelastically scattered doubles, and a 50% reduction in wavelength causes a 16-fold increase in the signal strength. Therefore, it would seem evident that a shorter wavelength laser is always the best option, but when we factor in the other two considerations, things get complicated. The biggest challenge with Raman spectroscopy is its relatively week signal strength compared to the fluorescence, and most complex molecules exhibit auto-fluorescence when excited with ultraviolet and visible light. As a result, most organic and biological samples must be excited in the near-infrared to avoid the Raman signal being drowned out by the fluorescence background. The strange dichotomy created by these competing effects, where on the one hand decreasing the wavelength will produce greater signal intensity while simultaneously increasing the background noise (auto-fluorescence) is the single most significant contributor to the reason why there are so many different laser wavelengths utilized in Raman spectroscopy.

RPMC offers a wide range of visible and near-infrared volume Bragg grating (VBG) external cavity diode lasers, which are ideal for Raman spectroscopy. For additional information including detailed technical specifications on these lasers click here or talk to one of our laser experts today by calling 1-636-272-7227.

 SHIPS TODAY
SHIPS TODAY